Synairgen Shares Down 4% in August – Time to Buy SNG Shares?
Please note that we are not authorised to provide any investment advice. The content on this page is for information purposes only.
The price of Synairgen shares has gone down 4% so far in August 2021 while the stock has retreated almost 3% so far this year following last year’s remarkable 2,500% advance.
Expectations about the potential approval of the firm’s SNG001 drug designed to treat COVID-19 and other conditions have continued to support Synairgen’s share price even though the firm has not made any recent announcements about further advancements in its Phase III trials.
Last year, the company’s Phase II trials for SNG001 produced positive results among a group of 101 patients. The study found that patients who received the drug had “greater odds of improvement on the OSCI scale” on day 15 and day 16 of the treatment. Moreover, no fatalities were reported among patients who received SNG001 compared to three deceases in the placebo group.
Moreover, the company has already commenced Phase III trials for this important drug and could soon reveal the results of this study.
Finally, Synairgen has been enrolled in the US government-s COVID vaccine and treatment development initiative called “Operation Warp Speed”, with the company’s activities now being partially financed by the Department of Health and Human Services of the country.
Could this stock become the next Moderna (MRNA)? In the following article, I’ll take a closer look at the latest price action while also analyzing the latest results from the firm’s trials to see what can be expected from Synairgen stock in the future.
67% of all retail investor accounts lose money when trading CFDs with this provider.
Synairgen Shares – Technical Analysis
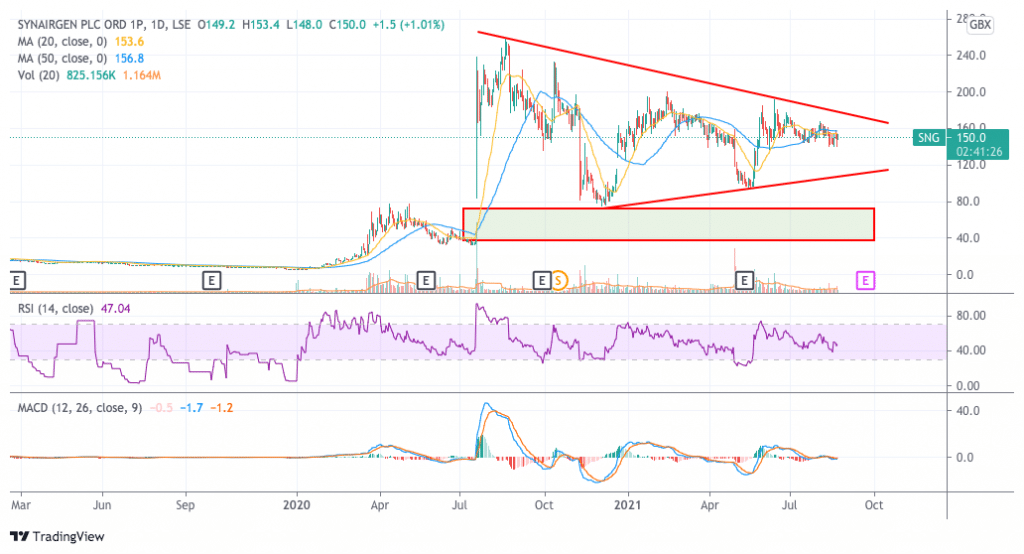
The latest price action in Synairgen shares has formed a typical consolidation triangle as market participants await the latest results of the company’s Phase III trials. The huge price gap left behind the 20 July 400%+ uptick continues to serve as support for the stock at the moment while future movements will likely be determined by news on the fundamental front.
From a historical standpoint, the stock is trading at a much more attractive entry price as it is sitting 42% below its 52-week high. However, a setback in the firm’s efforts to push its flagship COVID drug up the pipeline could result in a swift correction in the price.
Moving forward, the upper and lower bounds of the triangle remain the key resistance and support levels to watch and a tag of any of these thresholds could result in an opportunity to trade the stock with the expectation that the price will remain range-bound until further news come.
That said, traders should act cautiously by setting a stop-loss order on Synairgen shares to limit the damage that a sharp drop resulting from a disappointing development could have on their portfolio.
Synairgen Shares – Fundamental Analysis
Synairgen is a UK-based pre-revenue biotech company that has no marketable products. The company’s future revenue generation capacity is fully dependent on the approval of the products that are currently within its pipeline including the SNG001 COVID-19 treatment and another drug called LOXL2 designed to treat fibrotic diseases.
Currently, the SNG001 treatment is undergoing Phase III trials while the LOXL2 inhibitor has completed its Phase I trials.
The SNG001 treatment could also be indicated for treating other diseases apart from COVID-19 including asthma and chronic obstructive pulmonary disease (COPD).
In the past year, the company spent almost $24.3 million in research and development and has obtained the funds to perform its activities through the issuance of its common stock.
Moreover, by the end of 2020, the company had approximately $103 million in cash while its partnership with the United States government to perform its Phase III trial for SNG001 should cushion the impact that higher trial-related expenditures could have on its cash burn.
Moving forward, the results of the company’s Phase III “SPRINTER” trial will be crucial for the company. and two primary endpoints need to be met for the trial to be considered successful. These are related to the time it takes patients to recover in the 28 days that follow the administration of the treatment and the time it takes the hospital to discharge the patient.
First patient dosing has already commenced in January 2021 for this Phase III trial and initial results are expected to be revealed during the second semester of 2021.
Interestingly, analysts are already expecting that Synairgen will be granted approval for SNG001 this year and the company is forecasted to end 2021 with sales of around £146.5 million.
Moreover, sales are expected to climb to £583 million in 2022 and may continue to move higher in subsequent years if results from these trials are positive and the drug is approved for treating other conditions apart from COVID.
At its current market capitalization of £300 million, this stock does have the potential to become the next Moderna as long as the Phase III trial meets its primary endpoints.






